Your cart is currently empty!
Medical Writing Plan Template – ICH & GCP Compliant
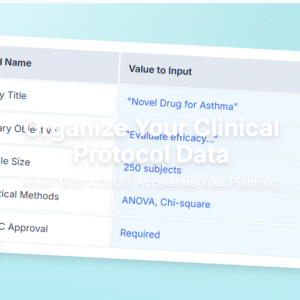
A professionally structured Medical Writing Plan Template tailored for clinical research teams, CROs, and freelance medical writers. This editable document complies with ICH E3, ICH GCP, and industry-standard SOPs. Perfect for Phase I–III clinical trials, this partially customized template provides the structure, regulatory references, and example content needed to save hours of formatting and regulatory…
Description
A professionally structured Medical Writing Plan Template tailored for clinical research teams, CROs, and freelance medical writers. This editable document complies with ICH E3, ICH GCP, and industry-standard SOPs.
Perfect for Phase I–III clinical trials, this partially customized template provides the structure, regulatory references, and example content needed to save hours of formatting and regulatory guesswork.
📦 What’s Included:
-
🗂️ Editable Word (.docx) template
-
📑 Fully structured Table of Contents
-
📋 Pre-filled example content in each section
-
🔒 ICH E3 and GCP compliance references
🎯 Who It’s For:
-
Clinical research professionals
-
Medical writing consultants & freelancers
-
CROs & biotech project teams
-
Regulatory affairs & quality teams
-
✅ Compatible with TMF and eCTD submissions
💡 Key Benefits:
-
✅ Saves 5–10 hours of formatting time
-
✅ Easy to customize for each protocol
-
✅ Ensures audit-ready document trail
-
✅ Helps align with SOPs and regulatory requirements
📥 Download Format:
-
Microsoft Word (.docx)
-
Instant digital download after purchase
🔄 License:
-
Personal & professional use
-
Unlimited projects
-
Not for resale or distribution
🔐 Refund Policy:
Due to the digital nature of this product, all sales are final.



Reviews
There are no reviews yet.